
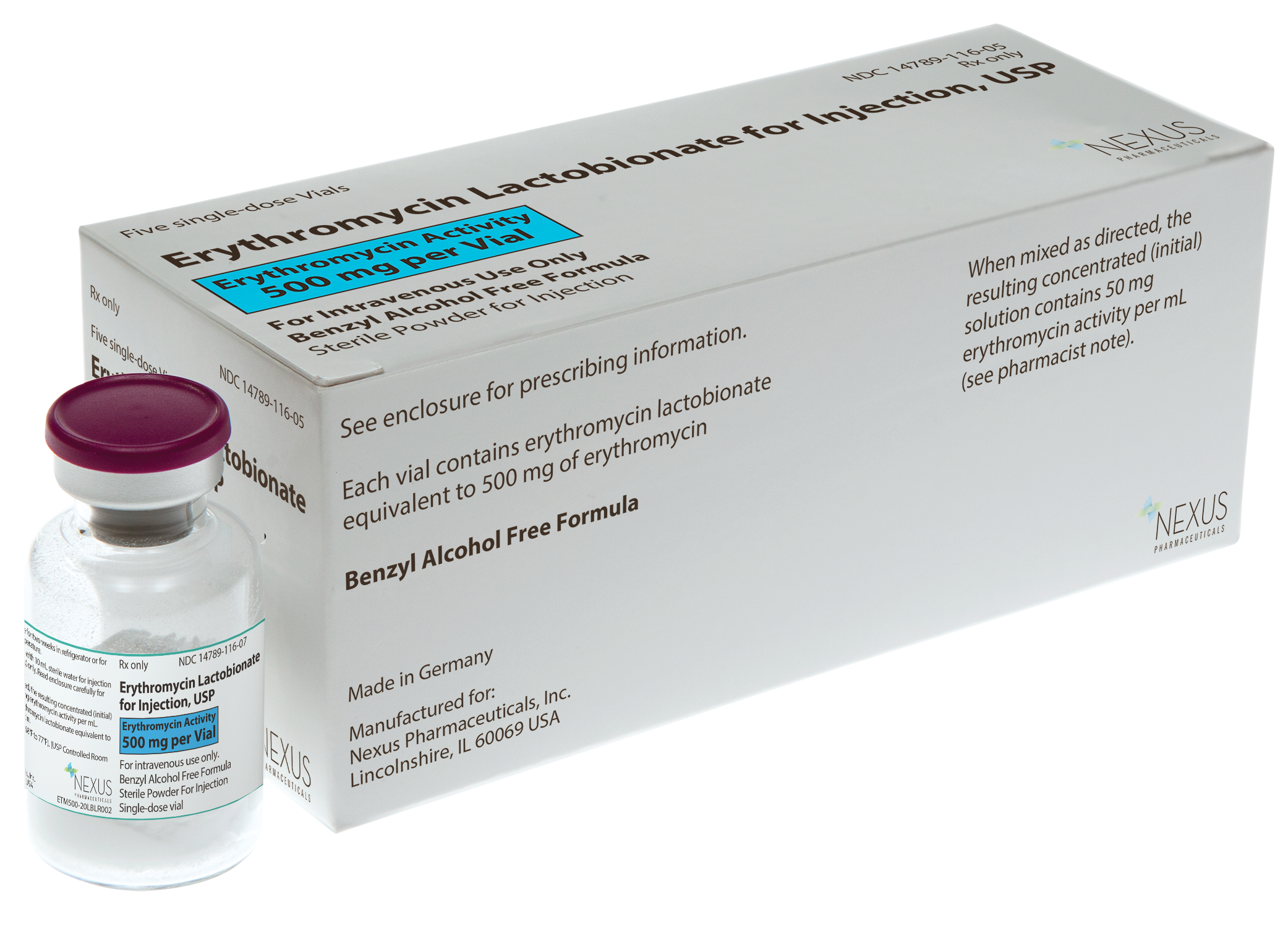
Erythromycin Lactobionate
for Injection, USP
First-to-Market AP Rated Generic to
Erythrocin™ Lactobionate*
Erythromycin Lactobionate for Injection, USP is indicated in the treatment of infections caused by susceptible strains of the designated organisms in diseases when oral administration is not possible or when the severity of the infection requires immediate high serum levels of erythromycin.
- Therapeutic Classification: Antibiotic
- Five units per carton for easier inventory management
- Latex free†
- Preservative-free
- Barcoded
†Vial closure is not made with natural rubber latex
Order directly through your wholesaler or from Customer Service at (888) 806-4606
Download a printable PDF product information sheet that includes ordering information and a scannable product barcode.

"Bringing this product to market showcases Nexus’ commitment to ending drug shortages in the US and solving today’s critical healthcare challenges."
Usman Ahmed, Chief Operating Officer
Nexus Pharmaceuticals
*ErythrocinTM Lactobionate is a resisted trademark of Hospira, Inc., a wholly owned subsidiary of Pfizer Inc.

